Immunoglobulin domain
The immunoglobulin (Ig) domain is found in a wide variety of proteins, in particular those involved in the immune response. This includes immunoglobulin molecules themselves (antibodies), T cell and B cell receptors, major histocompatibility (MHC) proteins (including beta-2-microglobulin), Fc receptors, as well as the CD4 and CD8 co-receptors.
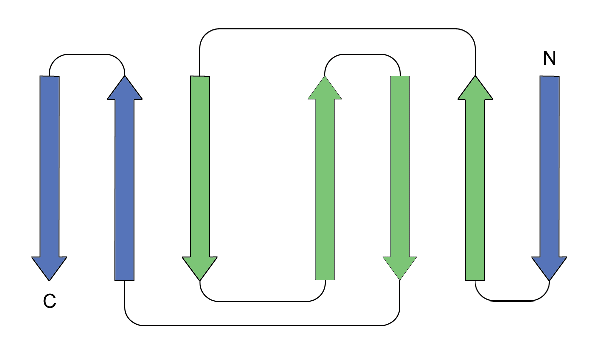
Each Ig domain is composed of around 110 amino acid residues, which make up seven beta strands, four of which make up one beta sheet whilst the other three make up another. The two sheets are linked by a disulphide bond. A greek key motif makes up part of the structure of the Ig domain, shown in green on the topological diagram below.
The IgG subclass of immunoglobulins are the major type of immunoglobulin in normal human serum. Each IgG molecule is made up of two heavy chains and two light chains; in turn each light chain is made up of two Ig domains, one variable (VL) domain and one constant (CL) domain, whilst each heavy chain comprises four Ig domains, three constant (CH1, CH2, CH3) domains and a single variable (VH) domain. One VH and one VL domain at the end of each Fab arm together determine antigenic recognition. An example of a IgG1 CH1 domain is given below (CATH domain ID 1l6xA02).
Ig-like domains are also found in domains not thought to be homologous to the domain found in the immunoglobulins, which have instead occurred by convergent evolution. The Ig fold, therefore, can be classified as a superfold .